Abstract
Background: Hereditary antithrombin deficiency (HAD) is a severe rare thrombophilia with an estimated prevalence of 0.02% to 0.2% in the general population. Patients with HAD have a high risk of developing arterial and venous thromboembolism (VTE). The purpose of ATHN 12: HAD Pilot Project is to collect data on participants diagnosed with HAD across the United States to better understand this rare thrombophilia and to develop guidelines regarding timing of screening for HAD, type of antithrombotic therapy, and indications for using antithrombin concentrate.
The ATHN 12 study is sponsored by the American Thrombosis and Hemostasis Network (ATHN). ATHN will leverage its existing data collection infrastructure and its ATHN-affiliated network of 146 hemostasis and thrombosis treatment centers in the United States to identify a cohort of approximately 100 patients with HAD who are receiving care in these centers.
Methods: We designed a national HAD registry to collect clinical, laboratory, and treatment data for participants with HAD with the goal of optimizing their management. The ongoing prospective HAD registry project (ATHN 12) was created in 2020 to identify a cohort of 100 adult and pediatric participants with HAD receiving care at thrombosis centers affiliated with ATHN. We collected information on demographics, laboratory data, thrombotic events, risk factors, antithrombotic therapy, and use of antithrombin (AT) concentrate during pregnancy and procedures.
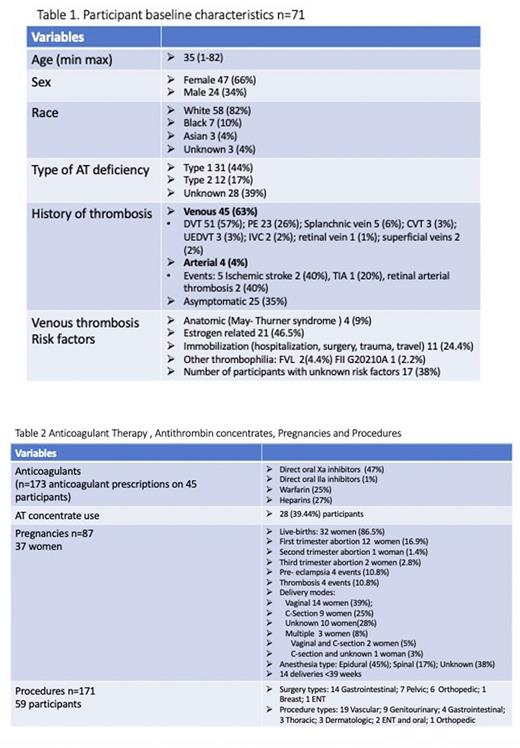
Results: 71 participants have been enrolled, 47 (66.2%) are female; mean age is 35 years (1-82); the majority 58 (82%) are White and 31 (43.6%) have type 1 HAD; 62 (87.32%) had a known diagnosis of HAD; 45 (63.38%) had a history of VTE (Table 1). The most common VTE locations included: lower extremity deep vein thrombosis 51 events (57%) and pulmonary embolisms 23 events (26%). 23 participants (51%) had recurrent VTEs. 4 participants (5.5%) had history of arterial thrombosis (AT) and 1 (25%) had a recurrent event. Of 45 participants, 17 (38%) had no other known risk factors for VTE; 21 (46.5%) were estrogen-related; 11 (24.4%) occurred after surgery, prolonged immobilization, hospitalization, travel, or trauma (Table 1). A total of 173 anticoagulants were prescribed on 45 (63.38%) participants (Table 2)
There were 87 pregnancies in 37 women. Low molecular weight heparin (LMWH) was administered in 46% of the pregnancies and in 3 women LMWH was switched to UFH between 36 to 38 weeks of gestation. AT concentrate was administered in 22 (25%) of the pregnancies before delivery and in 14 pregnancies 24 hours post-delivery. 31 women (36%) restarted anticoagulation post-delivery. The earliest initiation of anticoagulation was 6 hours after delivery. Overall, there were 34 (39%) pregnancy complications: 23 pregnancy losses mainly in first trimester, 4 thrombosis, 4 pre-eclampsia, and 6 other complications (Table 2).
59 participants (83%) had a total of 171 surgeries or procedures. The most frequent surgeries were gastrointestinal, and the most common procedures were vascular (Table 2). Overall, 28 (39.4%) have received AT concentrate without adverse events.
Conclusions: VTE was the most frequent clinical manifestation with DVT accounting for 57% of events and PE 26%. Oral factor Xa inhibitors were the most common anticoagulants prescribed. LMWH was administered in almost half of the pregnancies and AT concentrates in 25% of the pregnancies before delivery, and in 39.4% overall in pregnancies and procedures without reported adverse events.
Disclosures
Roberts:Novo Nordisk, Octapharma, Pfizer, Sanofi, Takeda, Genentech, Novartis: Consultancy; Takeda, Genentech; Speakers Bureau: Novo Nordisk, Sanofi, Takeda: Research Funding. Tarantino:OctapharmaUSA: Honoraria, Research Funding, Speakers Bureau; Grifols: Speakers Bureau; Pfizer: Research Funding. Recht:Oregon Health & Science University: Ended employment in the past 24 months; Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees; American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.